

Analysis of the legal regulation of Drug-Device Combination Products in China, the EU, and the U.S.
Foreword
With the continuous development of the drug and medical device industry and science and technology, more and more innovative Drug-Device Combination Products of drugs and medical devices (“Drug-Device Combination Products”, and each, a “Drug-Device Combination Product”) have appeared at home and abroad. Due to the complexity and diversity of Drug-Device Combination Products, practical issues in the registration and regulatory process at home and abroad are increasingly prominent. In this regard, some countries and regions including the EU and the U.S. have formed a complete set of regulations and guidance applicable to the Drug-Device Combination Products. In recent years, China's drug regulatory authorities have also closely followed the frontier of the development of Drug-Device Combination Products at home and abroad, drawing on the foreign experience in regulation to try to establish a scientific principle for determining which is suitable for China's Drug-Device Combination Products, so that scientific guidance and reasonable regulation can be carried out in the case of the increasing maturity of innovative Drug-Device Combination Products and the broad market prospects.
This article will analyze the regulation of Drug-Device Combination Products in China, the EU, and the U.S. based on the practice of legal regulatory and from the perspective of comparative law.
Definition of Drug-Device Combination Products
In China, according to the Notice on Matters Concerning the Registration of Drug-Device Combination Products (“the Notice”) issued by the National Medical Products Administration (“NMPA”) in July 2021, Drug-Device Combination Products refer to medical products composed of drugs and medical devices and produced as a single entity.
The EU and the U.S. do not have a clear definition of Drug-Device Combination Products, but according to the Regulation (EU)2017/745 (“MDR”) and Title 21 of the Code of Federal Regulations (“21 CFR”) for the regulation of Drug-Device Combination Products, generally speaking, a Drug-Device Combination Product is a product consisting of two or more different types of medical products (i.e., drugs, devices, and/or biologics in combination with each other).
Type of Drug-Device Combination Products
(一)China
China does not have a detailed classification of the Drug-Device Combination Products, and the Notice mainly divides them into (1) drug-led Drug-Device Combination Products which refer to Drug-Device Combination Products that drugs’ action is principal and (2) device-led Drug-Device Combination Products which refer to Drug-Device Combination Products that devices’ action is principal. The aforesaid Drug-Device Combination Products shall be subject to the application for registration and administration pursuant to the relevant requirements on drugs and medical devices, respectively.
(二)the EU
According to Article 1(8) and 1(9) of MDR, the Drug-Device Combination Products are divided into the following types for regulation in the EU:
(1) any device which incorporates a medicinal product that has an action ancillary to that of the device, shall be governed as a medical device;
(2) any device which incorporates a medicinal product whose action is principal and not ancillary to that of the device, shall be governed as a medicinal product;
(3) Drug-Device Combination Products comprised of a device intended to administer a medicinal product and a medicinal product that form a single integral product which is intended exclusively for use in the given combination and which is not reusable, shall be governed as a medicinal product; and
(4) other cases of Drug-Device Combination Products comprised of a device intended to administer a medicinal product and a medicinal product, shall be governed by the regulations of medical devices and medicinal products respectively regarding different parts.
(三)the U.S.
The scope and classification of Drug-Device Combination Products in the U.S. are more detailed and specific, and under 3.2(e) of 21 CFR, Drug-Device Combination Products include:
(1) A product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are physically, chemically, or otherwise combined or mixed and produced as a single entity (Single Entity Products);
(2) Two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and biological products, or biological and drug products (Combination Packaging Products);
(3) A drug, device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, device, or biological product where both are required to achieve the intended use, indication, or effect and whereupon approval of the proposed product the labeling of the approved product would need to be changed (Cross-labeled Products); and
(4) Any investigational drug, device, or biological product packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication, or effect (other Cross-labeled Products).
Regulatory Authorities of Drug-Device Combination Products
(一)China
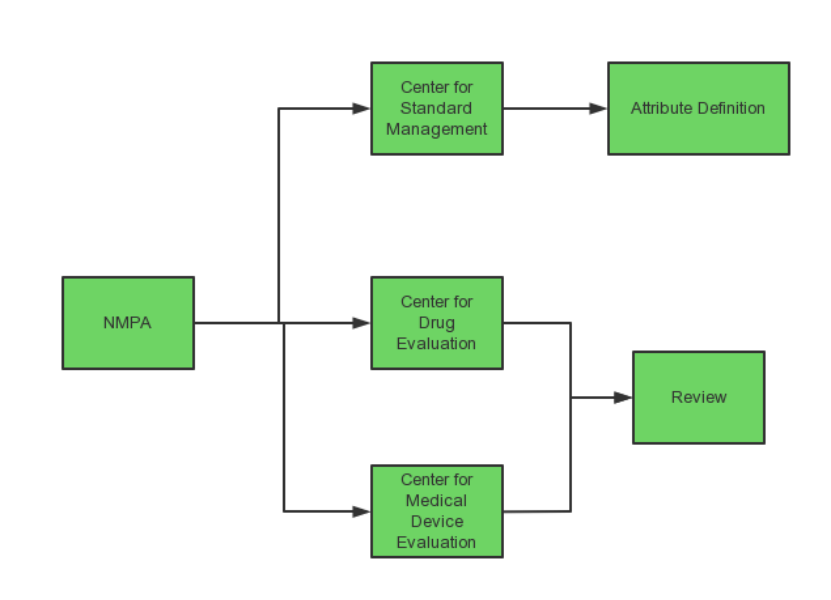
NMPA is mainly responsible for the regulation of Drug-Device Combination Products in China, consisting of (1) the Center for Medical Device Standard Management (“the Center for Standard Management”), responsible for the attribute definition of Drug-Device Combination Products; (2) the Center for Drug Evaluation and (3) the Center for Medical Device Evaluation, as the Lead Center, responsible for the review of Drug-Device Combination Products applied as drugs and as medical devices for registration respectively.
(二)the EU
In the EU, the attribute definition of Drug-Device Combination Products often involves regulatory authorities including national competent authorities of the Member States, the European Commission, and the European Medicines Agency (“EMA”), while the pre-marketing review and approval of Drug-Device Combination Products are subject to the regulation by EMA or National Competent Authorities responsible for medicinal products and Notified Bodies responsible for medical devices, depending on the product classification.
(三)the U.S.
The U.S. established the Office of Drug-Device Combination Products (“OCP”) at the Food and Drug Administration (“FDA”) in 2002 to be responsible for the classification of Drug-Device Combination Products, assignment of Lead Centers, and policy and regulation development. Besides, Drug-Device Combination Products’ pre-marketing review and post-marketing regulation involve different FDA centers, i.e., the Center for Drug Evaluation and Research (“CDER”), the Center for Devices and Radiological Health (“CDRH”), and the Center for Biologics Evaluation and Research (“CBER”) for collaboration.
Attribute Definition of Drug-Device Combination Products
(一)China
1.Determination Principle
The Notice provides principles for determining the Drug-Device Combination Product management attributes: (1) drug-led Drug-Device Combination Products shall be regulated under the drug regulations; while (2) device-led Drug-Device Combination Products shall be regulated under the medical device regulations.
2.Determination Procedure
According to the Notice, applicants should fully assess the attributes of their proposed Drug-Device Combination Products. If the management attributes of the Drug-Device Combination Products cannot be determined, the applicant shall apply to the Center for Standard Management for attribute definition of the Drug-Device Combination Products before registration application. Specific attribute definition application procedures and materials can refer to the “Attribute Definition Procedures for Drug-Device Combination Products” and “Attribute Definition Application Materials Requirements and Instructions for Drug-Device Combination Products” issued by NMPA at the same time as the Notice. The Center for Standard Management will review the application materials for the attribute definition of the Drug-Device Combination Products, give opinions on the attribute definition under the procedures, inform the applicant in the information system, and timely publish the results of the attribute definition on its official website.
(二)the EU
1.Determination Principle
The determination principle of the Drug-Device Combination Products’ management attributes in the EU is similar to that in China, and the Drug-Device Combination Products are divided into different types for regulation as mentioned above: drug-led Drug-Device Combination Products shall be governed under drug regulations and device-led Drug-Device Combination Products shall be governed under medical device regulations.
2.Determination Procedure
The determination procedure in the EU when it is difficult to determine the management attributes of Drug-Device Combination Products is more complex than in China. It should be the responsibility of the Member States to decide on a case-by-case basis whether or not a product falls within the scope of the regulation. When the Member States cannot find a clear basis or give a definitive conclusion, the European Commission should make a decision and is allowed to consult other EU agencies, such as the EMA.
(三)the U.S.
1.Determination Principle
In the U.S., a Drug-Device Combination Product is assigned to an Agency center that will have primary jurisdiction (i.e., the Lead Center) for that product’s regulation. Under section 503(g)(1) of FD&C Act, the assignment of a Drug-Device Combination Product to a Lead Center is based on a determination of which constituent part provides the primary mode of action (“PMOA”) of the Drug-Device Combination Product.
2.Determination Procedure
When the product classification or the designation of the Lead Center is uncertain or is under dispute, sponsors may submit a request for designation (“RFD”) or pre-request for designation (“Pre-RFD”) to OCP. Sponsors may propose the classification and/or assignment they believe should apply and OCP makes the final determination with input from the relevant Agency components. If OCP does not respond to an RFD within sixty (60) days of filing, the classification or assignment proposed by the sponsor will become the final determination. The information FDA needs for assessments and the processes for determinations can be found in “How to Write a Request for Designation” and “How to Prepare a Pre-Request for Designation” of the guidance for industry.
六、Review and Approval of Drug-Device Combination Products
(一)China
According to the Notice, the applicant can file drug or medical device registration applications with NMPA based on the result of the attribute definition and indicate “Drug-Device Combination Products” in the application form.
Drug-Device Combination Products applied as drugs are led by the Center for Drug Evaluation for review, while Drug-Device Combination Products applied as medical devices are led by the Center for Medical Device Evaluation for review. For Drug-Device Combination Products that need joint review, the registration application materials shall also be forwarded to another evaluation center for simultaneous review, and both centers shall collaborate to carry out the communication and consultation of the applied products, and issue review reports on the safety, effectiveness and quality control of the corresponding parts respectively, and clarify the evaluation conclusions. The Lead Center shall summarize and make an overall assessment and issue an overall evaluation conclusion which will be transferred to the corresponding divisions of NMPA for administrative approval.
(二)the EU
In the EU, the EMA or National Competent Authorities is responsible for the review and approval of medicinal products, while the Notified Body is responsible for the review and approval of medical devices. Therefore, Drug-Device Combination Products regulated as different types should apply to the corresponding competent authorities for registration.
the EU provides different review procedures for different types of Drug-Device Combination Products, specifying that: (1) for Drug-Device Combination Products regulated as drugs, the Notified Body will provide an assessment of the device part; and (2) for Drug-Device Combination Products regulated as medical devices, the Notified Body will consult the competent authority for the drug part. Specific requirements include:
1.Drug-Device Combination Products Regulated as Drugs
Such Drug-Device Combination Products shall comply with the General Safety and Performance Requirements (“GSPR”) set out in Annex I of MDR concerning the safety and performance of the device part. The marketing authorization dossier shall include, where available, the results of the assessment of the conformity of the device part with the relevant GSPR contained in the manufacturer's EU declaration of conformity or the relevant certificate issued by a Notified Body allowing the manufacturer to affix a CE marking to the medical device.
2.Drug-Device Combination Products Regulated as Medical Devices
Drug-Device Combination Products that medicinal products have an ancillary action are classified as class III medical devices under Annex VIII Rule 14 of MDR and shall meet the requirements of MDR. Before issuing an EU technical documentation assessment certificate, the Notified Body shall, having verified the usefulness of the medicinal product as part of the device and taking account of the intended purpose of the device, seek a scientific opinion from the competent authorities designated by the Member States or from the EMA on the quality and safety of the medicinal product including the benefit or risk of the incorporation of a medicinal product into the device. The medicinal products authority consulted shall provide its opinion to the Notified Body within two hundred and ten (210) days of receipt of all the necessary documentation. The Notified Body shall give due consideration to the views expressed in the scientific opinion when making its decision. The Notified Body shall not deliver the certificate if the scientific opinion is unfavorable and shall convey its final decision to the medicinal products authority consulted.
(三)the U.S.
Different types of Drug-Device Combination Products are classified according to their attributes for pre-marketing applications as drugs, medical devices, or biological products respectively. The departments responsible for the pre-marketing review include the CDER, the CDRH, and the CBER. After OCP classifies the products based on the application materials:
(1) the CDER is responsible for the review of drug-led Drug-Device Combination Products;
(2) the CDRH is responsible for the review of device-led Drug-Device Combination Products; and
(3) the CBER is responsible for the review of biologic-led Drug-Device Combination Products.
After the Lead Center is determined, it has primary jurisdiction over the pre-marketing review and post-marketing regulation of the product. Generally, the review of a Drug-Device Combination Product shall include all information about the entire product, and the Lead Center completes the review through consultation or collaboration with other relevant centers and OCP, with the relevant centers evaluating the safety and effectiveness of each constituent part and the Lead Center performing a combination or coordinated analysis of the evaluation results and making decisions. During the review process, each center shall maintain standardized and consistent procedures and standards for product evaluation.
七、Post-marketing Regulation of Drug-Device Combination Products
(一)China
China's post-marketing regulation of Drug-Device Combination Products is still in accordance with the regulatory modes of drugs and medical devices. Up to now, there are no relevant guidance documents issued or specific requirements in China. Considering the special nature of the Drug-Device Combination Products, the EU and the U.S. have made more detailed rules regarding the post-marketing regulation of them, which is worthy of our reference.
(二)the EU
In the EU, manufacturers of Drug-Device Combination Products are required to meet the requirements of EMA for active post-marketing surveillance and post-marketing safety reporting and prepare post-marketing surveillance plans. In addition, the post-marketing surveillance requirements provided in Annex XIV of MDR are also applicable to Drug-Device Combination Products regulated as medicinal products.
(三)the U.S.
The FDA has issued corresponding guidance documents for post-marketing quality control and post-marketing safety control of Drug-Device Combination Products.
On the one hand, for the post-marketing quality control of Drug-Device Combination Products, FDA requires to meet both drug and medical device CGMP requirements and requires companies to establish and maintain appropriate and effective personnel organizational structures, quality management systems, and control procedures to ensure that quality systems can meet the established quality policies and objectives.
On the other hand, for the post-marketing safety regulation of Drug-Device Combination Products, FDA has established a consistent and efficient post-market safety reporting (“PMSR”) system. Reporting requirements vary according to the characteristics of drugs, medical devices, and biologics, but the FDA Lead Center reviews and consults with OCP and other centers on PMSR information.
八、结语 Conclusion
China, the EU, and the U.S. have common ground for the regulation of Drug-Device Combination Products, but there are also some differences.
First, the definitions and principles of attribute definition of Drug-Device Combination Products are similar in the three areas, and in general, the corresponding PMOA is used to determine the management attributes of the products.
Second, the EU and the U.S. have border ranges and more detailed classifications of Drug-Device Combination Products. For example, the biologics are also included in the regulatory scope of the combination of products in the U.S., and in addition to single entity products, combination packaging products and cross-labeled products are also included. In contrast, the range of Drug-Device Combination Products is relatively narrow in China, and the classification is not detailed either.
Finally, in terms of regulatory documents and systems, on the one hand, the EU and the U.S. have more scientific and mature management of Drug-Device Combination Products, with clear policies or Guidelines in every aspect and procedure, while China's provisions on Drug-Device Combination Products are still relatively limited and not comprehensive, as the Notice is just a simple elaboration of the Drug-Device Combination Product registration-related matters, and other more specific guidance documents and supporting regulations have not been developed so that the legal issues of Drug-Device Combination Products cannot be fully covered. On the other hand, China’s post-marketing regulation of Drug-Device Combination Products is still blank, simply following the independent regulatory modes of drugs and medical devices, while the EU and the U.S. have gradually developed a series of regulations to guide the post-marketing regulation considering the special characteristics of Drug-Device Combination Products.
In conclusion, in order to adapt to the complexity and specificity of the Drug-Device Combination Products, China could draw on international advanced experience in regulation, study and establish suitable management modes of the Drug-Device Combination Products, and consider further refining the classification and clarifying the regulatory scope of Drug-Device Combination Products, and further introducing specific guidance documents or regulations based on Drug Administration Law (Revised in 2019), Regulation on the Supervision and Administration of Medical Devices (Revised in 2021) and the Notice to improve the supporting measures and the construction of legal systems for the management of Drug-Device Combination Products and implement effective and scientific regulation in the whole life cycle of the Drug-Device Combination Products.
* Comments:
[1] Whereas:(8) of MDR.
[2] 3.2(m) of 21 CFR: “the PMOA of a Combination Product is the single mode of action (drug, device, or biological product) expected to make the greatest contribution to the overall intended therapeutic effects of the Combination Product” .
[3] Article 117 of MDR.
[4] Annex IX Article 5.2 of MDR.
[5] U.S. FDA. Guidance for Industry and FDA Staff: Current Good Manufacturing Practice Requirements for Combination Products.
[6] U.S. FDA. Compliance Policy for Combination Product Post-Marketing Safety Reporting: Immediately in Effect Guidance for Industry and Food and Drug Administration Staff.
来源于:网络 ,北京龙惠科技整理
