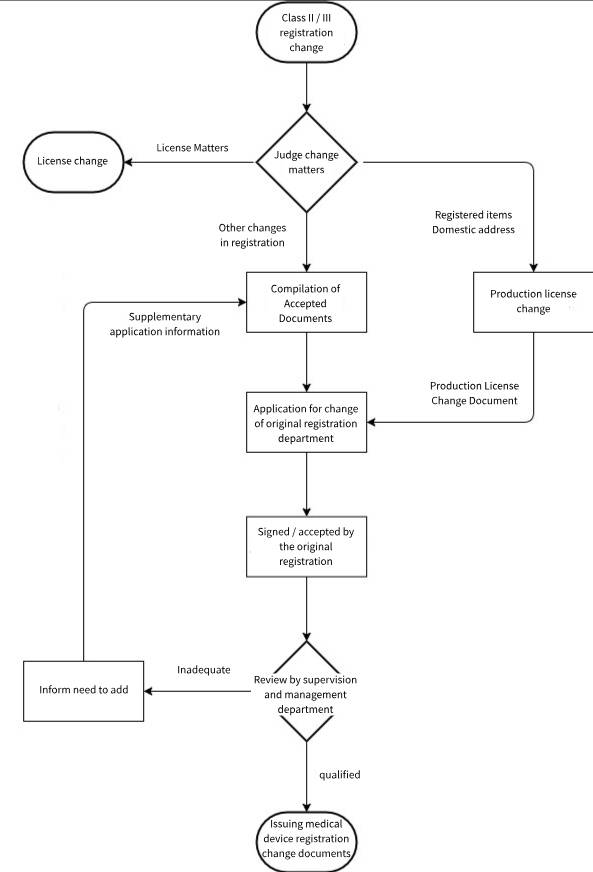
Change of registration items:
1. The name and address of the registrant;
2. The name of the agent and the address of the address of the residence;
3. The production address of domestic medical equipment;
License changes:
1. Product name;
2. model;
3. Specifications;
4. Structure and composition;
5. Scope of application;
6. product technical requirements;
7. The production address of imported medical equipment;